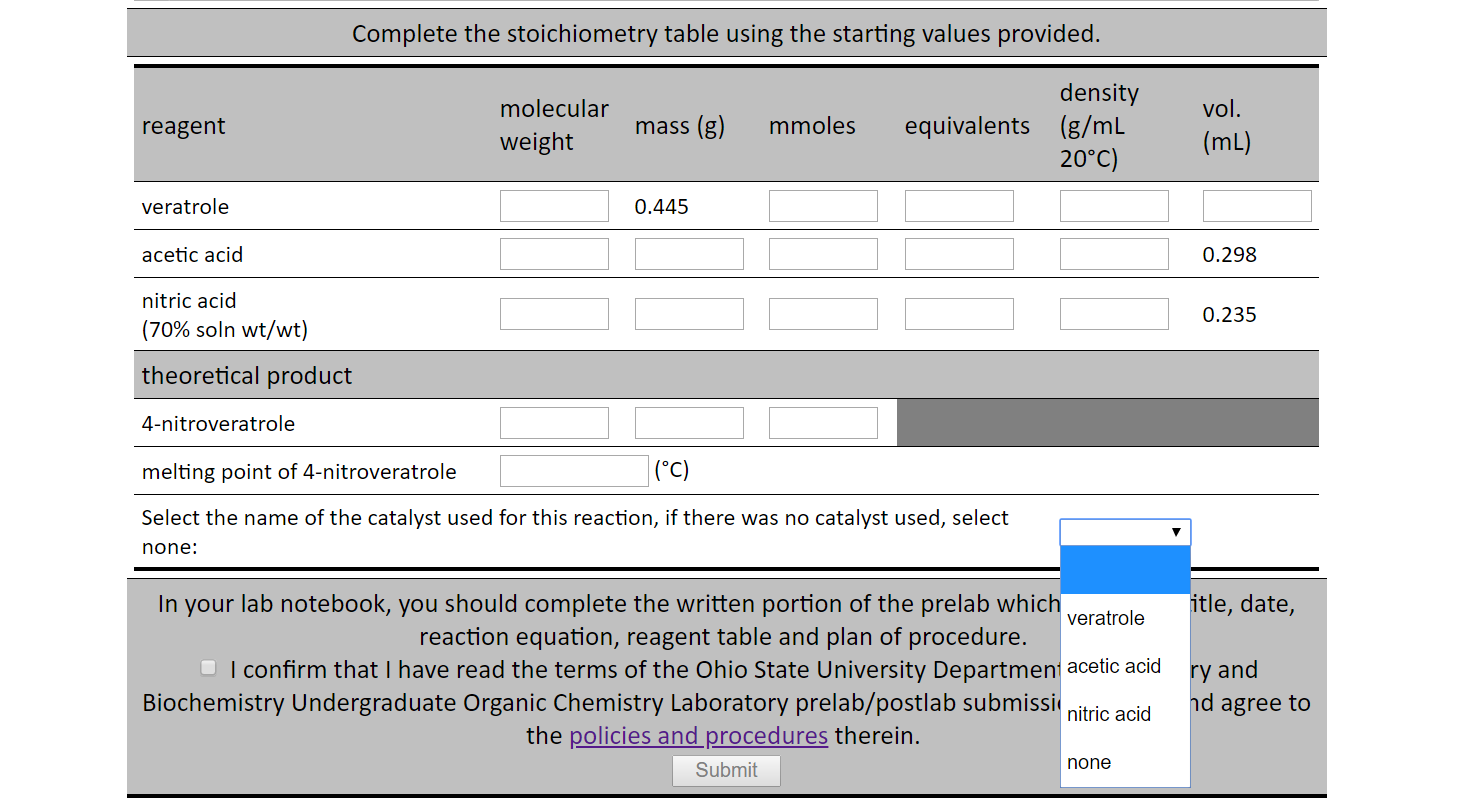
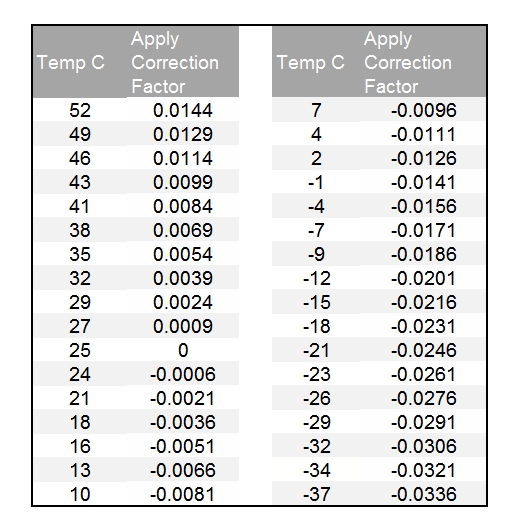
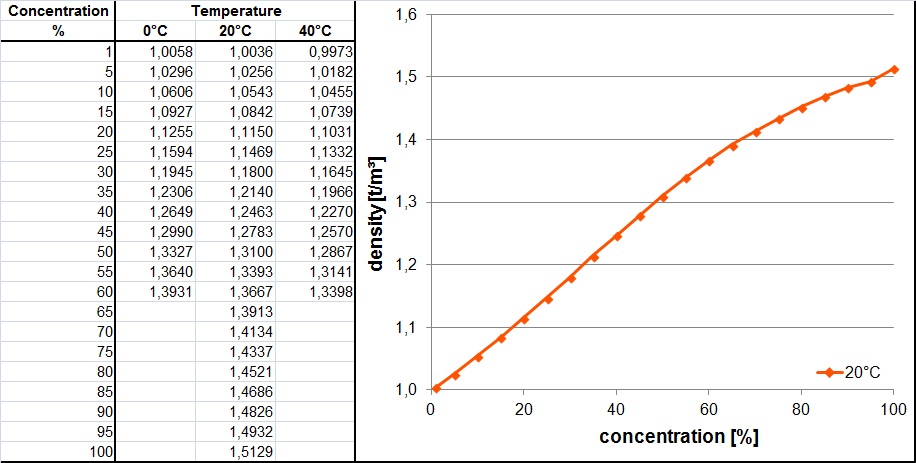
Vapor pressures of concentrated nitric acid solutions in the composition range 83 to 97 percent nitric acid 0 to 6 percent nitrogen dioxide, 0 to 15 percent water, and in the temperature
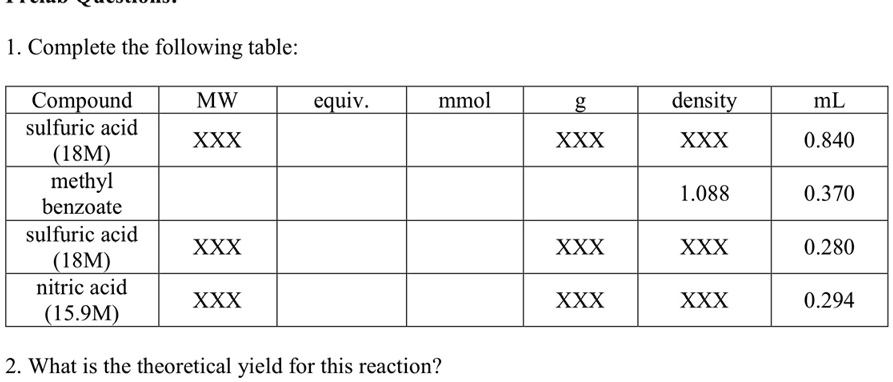
SOLVED: Compound MW equiv mmol density mL sulfuric acid (18M) XXX XXX XXX 1.840 XXX methyl benzoate XXX XXX XXX 1.088 XXX sulfuric acid (18M) XXX XXX XXX 0.370 XXX nitric acid (
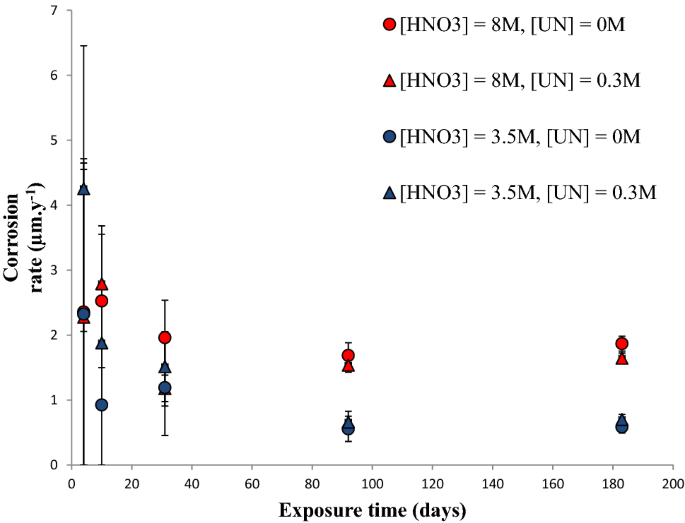
Corrosion studies of stainless steel 304 L in nitric acid in the presence of uranyl nitrate: effect of temperature and nitric acid concentration | SN Applied Sciences
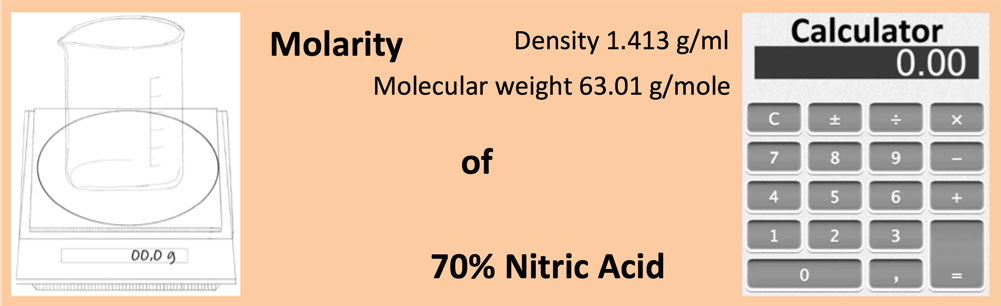
Calculate the concentration of nitric acid in miles per litre ina sample which has a density 1.41g/mL and the mass percent of nitric acid in it being 69percent.

Nitric and Sulfuric Acid Solubility in Dense Phase CO2 | Industrial & Engineering Chemistry Research

Calculate the concentration of nitric acid in moles per litre in a sample which has a density - YouTube