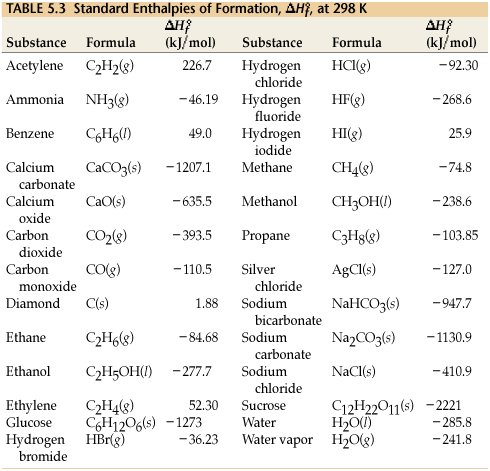
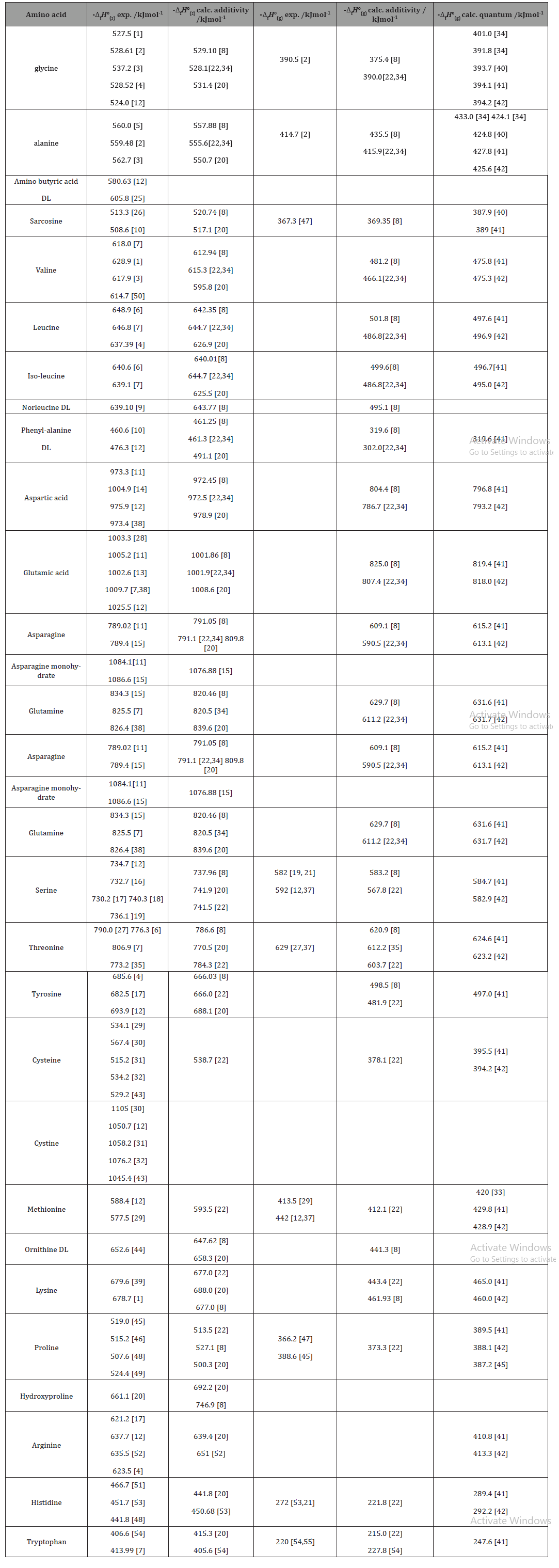
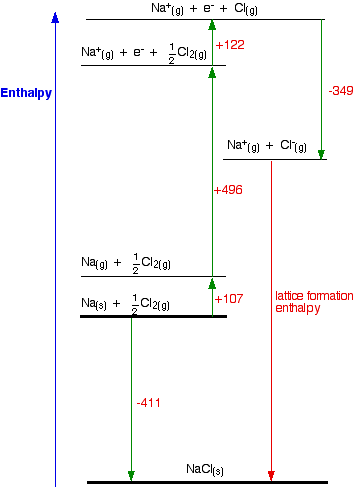
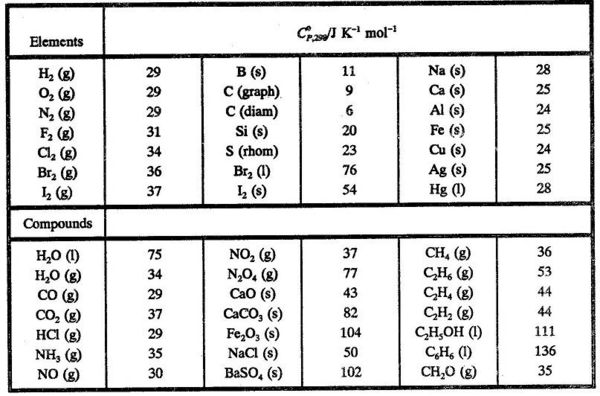
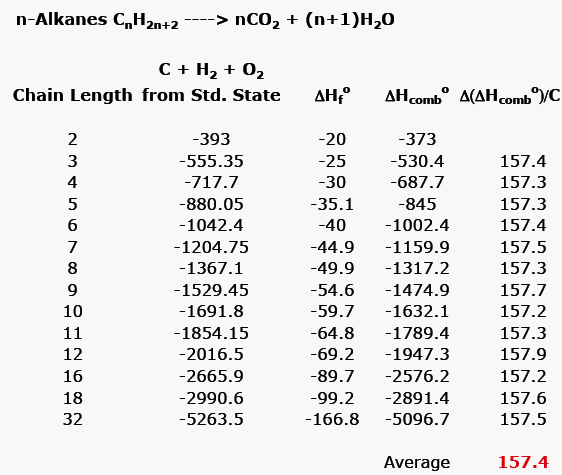
2009, Prentice-Hall, Inc. Enthalpies of Formation An enthalpy of formation, H f, is defined as the enthalpy change for the reaction in which a compound. - ppt download

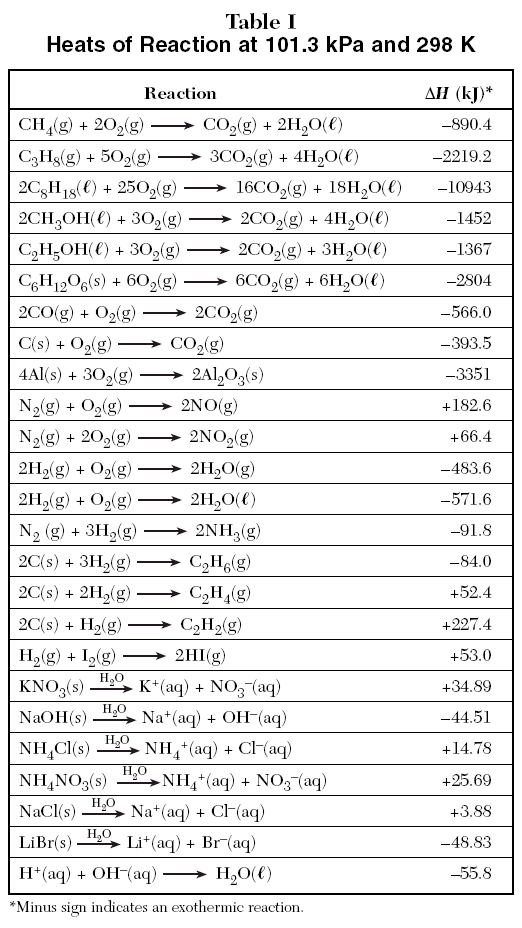
Table 6 from Structure and heats of formation of iodine fluorides and the respective closed-shell ions from CCSD(T) electronic structure calculations and reliable prediction of the steric activity of the free-valence electron
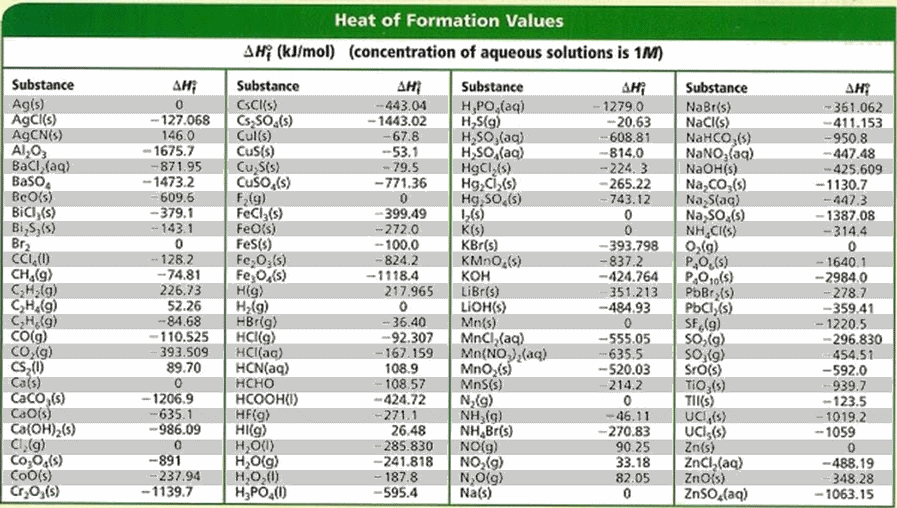
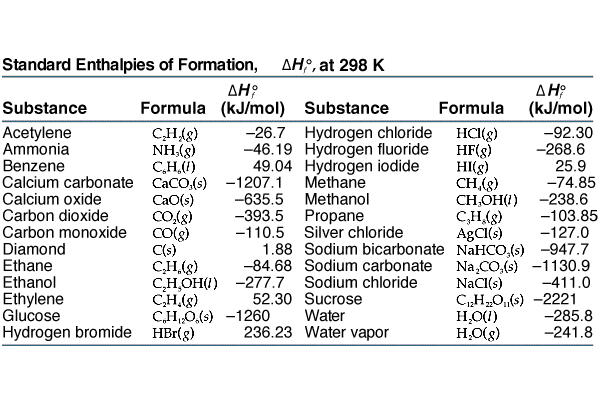
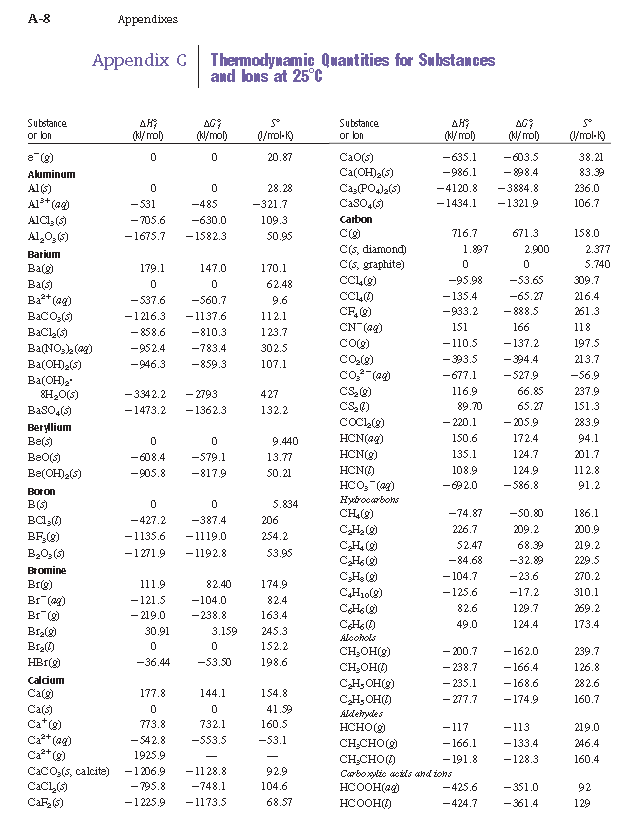
![Appendix D. Heats of Formation and Combustion - Basic Principles and Calculations in Chemical Engineering [Book] Appendix D. Heats of Formation and Combustion - Basic Principles and Calculations in Chemical Engineering [Book]](https://www.oreilly.com/api/v2/epubs/9780132885478/files/graphics/appd-tab-d1a.jpg)
Appendix D. Heats of Formation and Combustion - Basic Principles and Calculations in Chemical Engineering [Book]
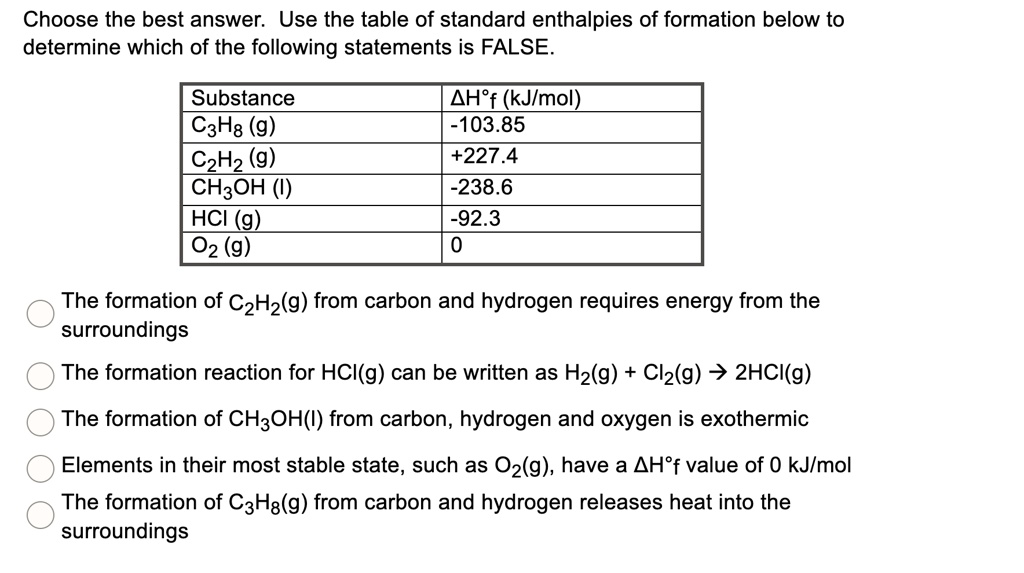
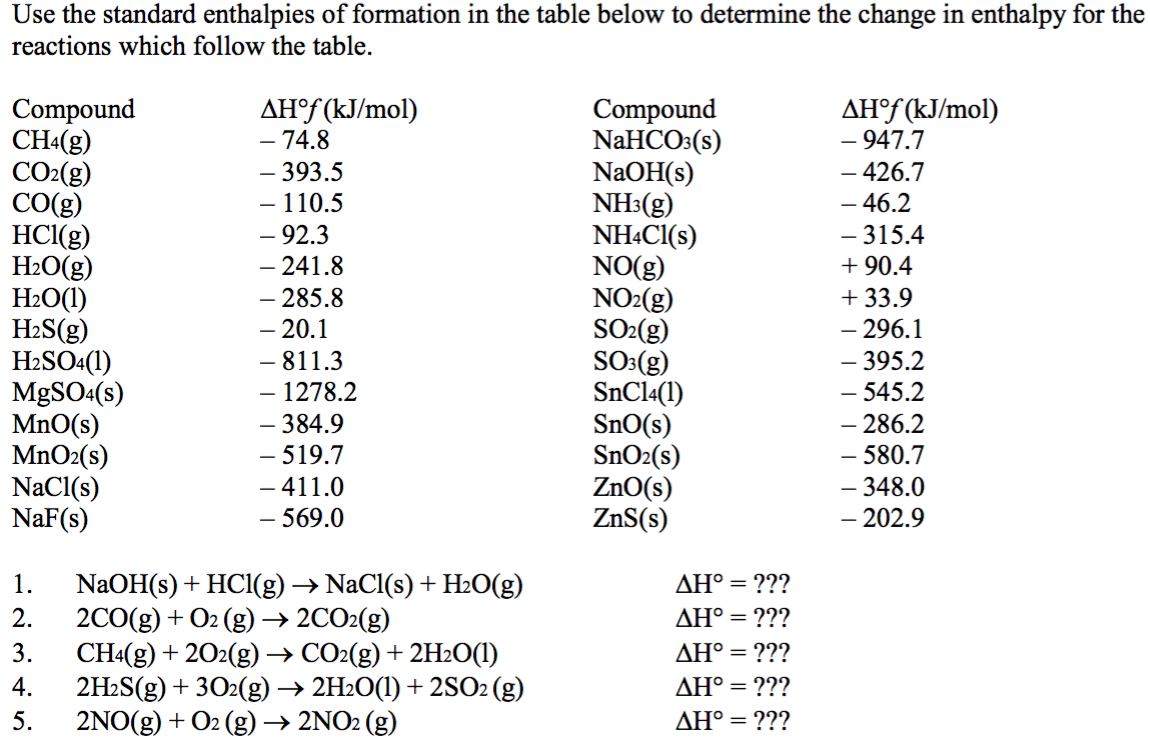
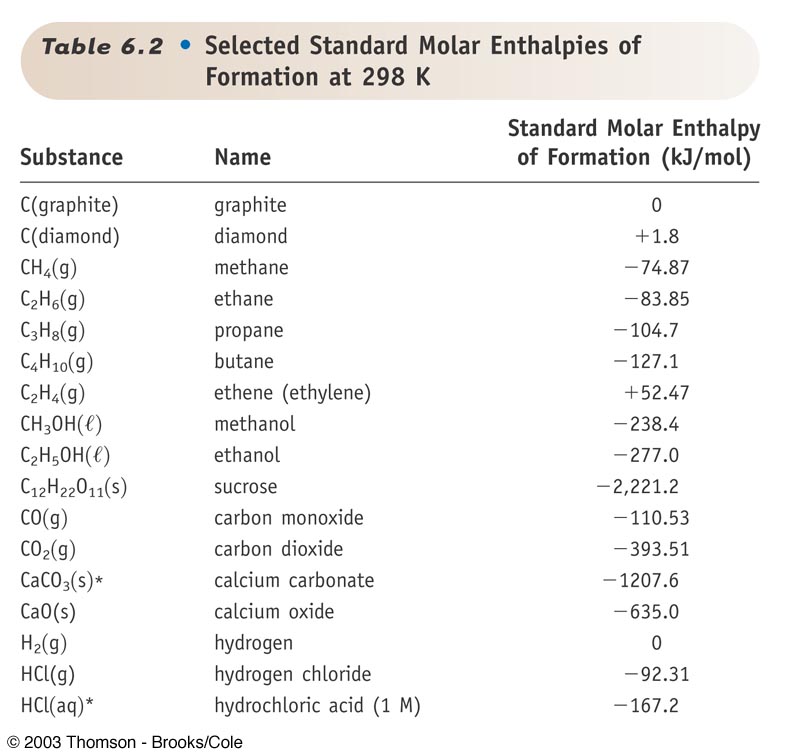
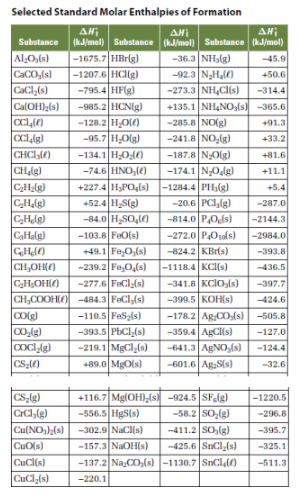
SOLVED: Choose the best answer: Use the table of standard enthalpies of formation below to determine which of the following statements is FALSE. Substance C2H2 (g) C2H4 (g) CH3OH HCl O2 (g)

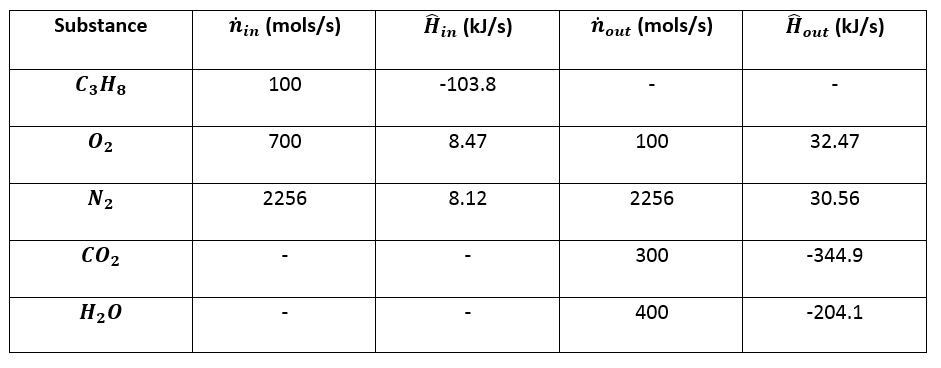
The question asks what is Using the provided table and the equation below, determine the heat of formation - brainly.com